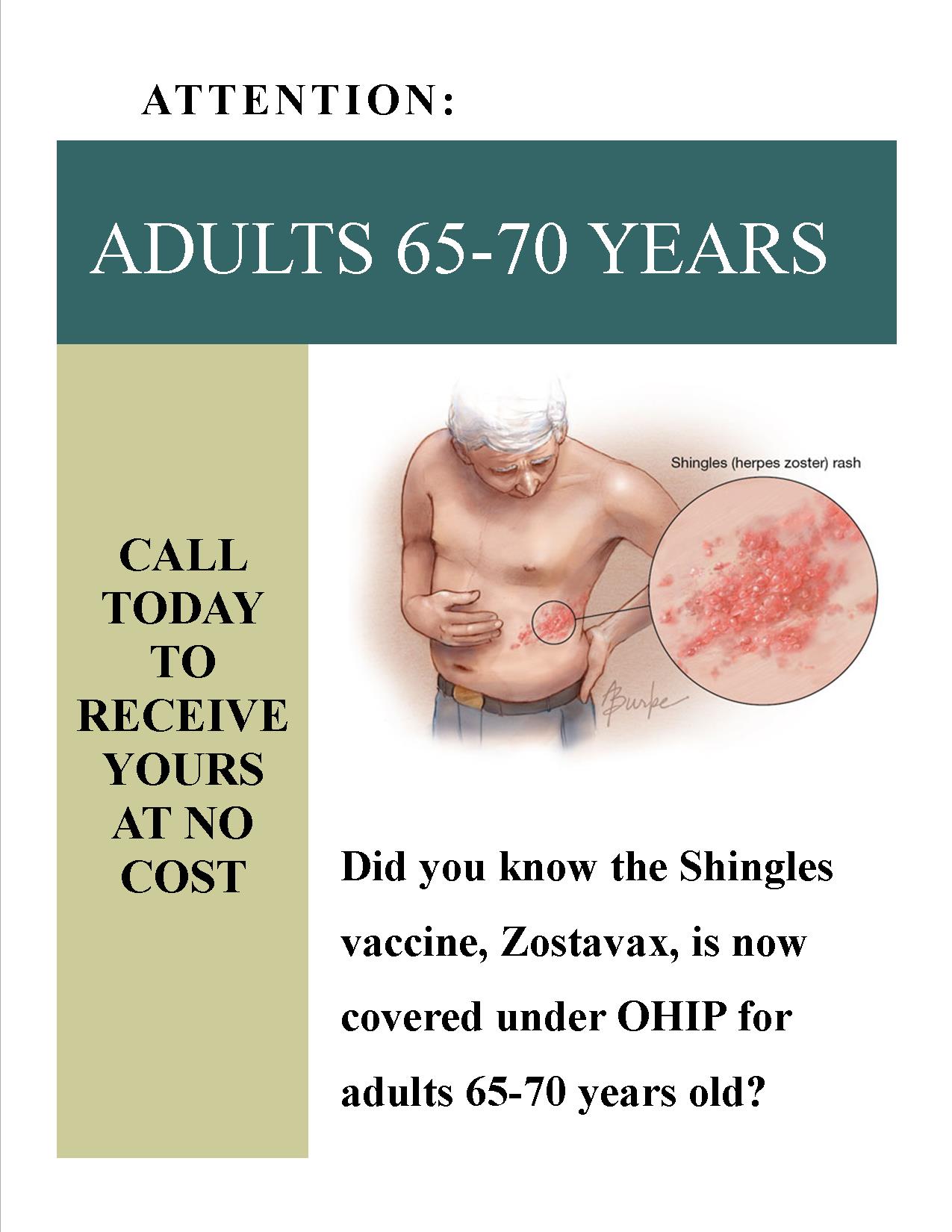
Shingles Vaccine (Zostavax II) Fact Sheet
What is Zostavax?
Zostavax is a new publicly funded vaccine for Shingles that is covered under OHIP for seniors aged 65-70 years old.
How is the vaccine given?
The vaccine is given in the upper arm, in the subcutaneous tissue. It can be given at the same time as any other vaccine such as the influenza vaccine or Tetanus booster.
There is no recommendation for people to receive a booster dose of Zostavax.
Are there any side effects?
The most common side effects are mild and can include injection site pain, swelling or redness. Other side effects that have reported include: a hard lump, itching, warmth, and bruising at the injection site. Headache and pain in an arm or leg have also been reported.
Who should not receive the vaccine?
- People who are immunocompromised (acute and chronic leukemias, lymphoma, conditions affecting the bone marrow or lymphatic system, immunosuppression due to HIV/AIDS) should not receive the vaccine.
- People who are on immunosuppressive therapy (including high-dose corticosteroids)
- People with a history of anaphylaxis after previous administration of the vaccine
- People with proven immediate or anaphylactic hypersensitivity to any component of the vaccine or its container, including gelatin or neomycin
- Pregnant individuals, diagnosis of active untreated tuberculosis, active herpes zoster
- People who have had an episode of shingles within the last year
Can you receive the vaccine if you have not had chickenpox previously?
Yes. The vaccine should be given whether or not the person has had varicella (chickenpox) illness due to the fact that nearly all Canadians have had exposure even if they cannot recall a diagnosis of Varicella illness.
Yes. The vaccine should be given whether or not the person has had varicella (chickenpox) illness due to the fact that nearly all Canadians have had exposure even if they cannot recall a diagnosis of Varicella illness.
